Types of Molecular Models
On this page:
- Ball and spoke models
- Space-filling models
- Crystal lattice models
The staff of the Whipple Museum are saddened to learn of the passing of longtime friend of the Museum Francis Hookham, who died peacefully on March 25th. Francis is known to many of our visitors through his much-loved collection of pocket electronic calculators, which he donated to the Museum in 1988. The collection was begun in 1975, just as electronic calculators were passing from expensive high-end scientific tools to their present status as disposable everyday technology. Hookham recognized the need to preserve early examples before they disappeared, eventually amassing over 450 different types before offering his entire collection to the Whipple. Since that time, Hookham’s calculators have been one of the Museum’s most popular displays. They continue to attract an enthusiastic response from visitors to our newly redisplayed Learning Gallery, where a selection remain on show.
While the Whipple Museum remains closed due to the covid-19 pandemic, visitors can learn more about Hookham’s collection in the Explore section of the Museum’s website.
The staff of the Whipple Museum pass on their sincere condolences to the Hookham family, and their thanks once again for Francis’s most generous donation.
Ball and spoke models
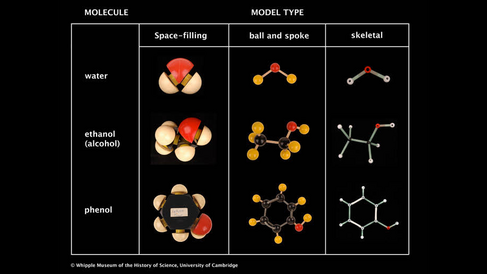
Ball and spoke models are a common way of representing molecular structures. Each atom is represented by a coloured ball that is joined to other atoms using spokes to represent the bonds between them. This type of model emphasises the bonding between atoms.
Showing how atoms bond together
Each wooden ball has a number of holes drilled into it. These correspond with the number of bonds that the atom can form with other atoms. The number of bonds that can be made depends on how many electrons an atom has, which varies depending on the element. For example, oxygen atoms can make two bonds, but hydrogen atoms can only make one.
The chemist Wilhelm August Hofmann first used coloured balls to represent the elements around 1865. John Dalton, who in the 19th century made great advances in thinking about the structure of atoms, used ball and spoke models in his lectures. Some of his models and diagrams of atoms are displayed in the Science Museum in London.
Other 'open' models: skeletal models
Skeletal models are similar to the ball and spoke kind; they are both classified as 'open' structures, as opposed to the 'closed' space-filling type. In skeletal models, the atoms are not shown as spheres. Instead the atoms are assumed to be at the intersection of two or more rods, which represent the bonds. The main advantage of skeletal models that is easy to measure angles and dimensions due to their open structure. The Whipple Museum has a set of Orbit skeletal models, shown in Image 2.
Space-filling models
Space-filling models give a representation of the size and shape of the whole molecule, showing (relatively) how much space each atom occupies. Space-filling models were first designed by H. A. Stuart in 1934.
Model features
Chemistry students use space-filling models to help when visualising whether the shape of certain bulky structures will prevent them reacting with other molecules. However, space-filling models make it difficult to see how the atoms bond together and prevents seeing the structure of the whole molecule clearly. Ball and spoke representations are much better for showing this information.
Space-filling models use a measurement known as the van der Waals radius to give the accurate size of each type of atom, based on the density of electrons around them.
Courtauld space-filling models by Griffin and George
This set of space-filling models in the Whipple Museum's collection was made by Griffin and George, a company that designed mass-produced models for students learning chemistry. Griffin and George's Courtauld Atomic Models set is extremely well known amongst students of the period. It was developed from the designs of Dr. G. S. Hartley of Courtaulds Ltd. in 1952 and underwent several improvements over the next fourteen years. The company Griffin and George had this name only between 1954-1957, so these sets of models can be closely dated to this time.
The models themselves are made of a rigid plastic, with a colour scheme that is different from the recommendations set out by the Institute of Physics. Instead, the colours of these models were chosen for how good they would look in black and white photographs.(1) The set comes with scale cards by Gallenkamp for estimating the size of the molecules (20mm equivalent to 0.1nm).
Packing structure models
Models that represent how atoms pack together can be similar in type to the space-filling kind. Atoms can be packed together in several different arrangements, and models are used to represent these arrangements. In the Museum's collection are models that demonstrate hexagonal close packing and cubic close packing.
These particular models are classified as the space-filling type because they model the atoms as hard spheres that are in contact with one another. Although this is not an entirely realistic way to think about atoms, it provides a clear way of visualising the arrangement.
Crystal lattice models
Molecular model kits are designed to be re-used; models can be built and then taken apart again, but chemists often make permanent models of molecular structures for demonstrations or teaching. This crystallographic model of the metal beta-manganese was made by Mr C. E. Chapman, Chief technician of the Crystallography Department, part of the Cavendish Laboratory at the University of Cambridge, in about 1952. It shows how the atoms of manganese are arranged at high temperatures.
The structure of crystals
A crystal lattice is a repeating pattern of atoms in a solid. It is an ideal representation of how atoms are bonded together and where they are situated with respect to their neighbours within the solid.
This crystal lattice model (Image 5) shows the distances between atoms of manganese and their geometrical arrangement within the crystal structure. The black wire cube represents the smallest possible cube that describes the general arrangement of atoms in the crystal lattice. It is referred to in crystallography as the unit cell.
Beta-manganese
In many metals all of the atoms are the same distance apart and surrounded by 12 other atoms (much like the arrangement of stacks of oranges in supermarkets). In manganese however, for reasons not fully understood, this is not the case. At room temperature some of the distances are shorter than others. This makes the arrangement of managese atoms in the crystal lattice more complicated than most other metals.
When metallic manganese is heated the crystal lattice undergoes changes in its structure before the metal melts. These different states are known as allotropes, and are named alpha, beta, gamma and delta to distinguish them. Between temperatures of about 700 to 1100 degrees centigrade, manganese exists in the beta state. As in the alpha state, not all the atoms are the same distance apart; but further, the atoms exist in two different geometrical arrangements (shown as red and green balls in Images 5 & 6). This does not mean that they are a different kind of atom, just that their neighbouring atoms are arranged differently.
Students of crystallography often find it difficult to understand the concepts of different geometrical sites in a crystal lattice, this problem is increased in a complicated crystal lattice such as beta-manganese. C. E. Chapman made this model especially to help a student visualise the arrangement of such sites. Models such as this are still used in teaching today.
References
- Griffin et al, 'Courtauld atomic models', Journal of Scientfic Instruments Vol. 32 (1955), 195-195.
Ruth Horry, 'Ball and spoke models', Explore Whipple Collections, Whipple Museum of the History of Science, University of Cambridge, 2006.
Ruth Horry, 'Space-filling models', Explore Whipple Collections, Whipple Museum of the History of Science, University of Cambridge, 2008.
James Hyslop, 'Crystal lattice models', Explore Whipple Collections, Whipple Museum of the History of Science, University of Cambridge, 2008.
